The market study done by Fact.MR gives exclusive information about how the market will grow. The study identifies crucial trends that are determining the growth of Global eCOA market. This newly published report sheds light on vital dynamics, such as the drivers, restraints, and opportunities for key market players as well as emerging players associated with the production and supply. The latest report by Fact.MR provides detailed Market Analysis of Global eCOA
The report offers actionable and valuable market insights of Global eCOA. The latest report by Fact.MR provides details on the present scenario of the market across various regions along with the historic data and forecast of the market. The report also includes information on the sales and demand of Global eCOA Market across various industries and regions.
To remain ‘ahead’ of your competitors, request for a sample – https://www.factmr.com/connectus/sample?flag=S&rep_id=4042
The latest Fact.MR study indicates healthcare facilities, contract research organizations, and educational & research institutes collectively spent ~US$ 1,950 Mn on eCOA, eSource and clinical trials solutions in 2018. The eCOA market is envisaged to witness substantial traction through 2019, with gains primarily driven by the growing penetration of digitization in healthcare sector and substantial increase in pharma spending on clinical trials.
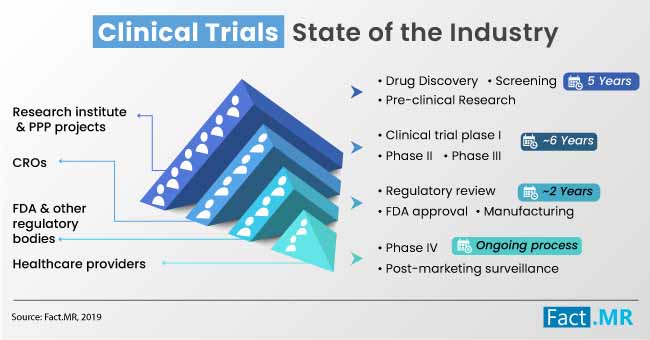
Rapidly expanding deployment of clinical trial solutions, which contributed to ~40% of the total spending on eCOA, eSource, and clinical trials in 2018, will continue to appeal a widening bandwidth of institutions through 2029, says the report.
Need more information about Report Methodology? Click here- https://www.factmr.com/connectus/sample?flag=RM&rep_id=4042
Spending on eCOA Set to Quadruple
The Fact.MR study opines that though the deployment of clinical trial solutions overshadowed the use of Electronic Clinical Outcome Assessment (eCOA), eSource, and EDA in 2018, the market is envisaged to witness a range of changes during the foreseeable period. Growing penetration of Bring Your Own Device (BYOD) trend in clinical trials is highly likely to pace up the use of eCOAs, such as ePROS, ClinROs, ObsROs, and PerfORs across wide range of pharmaceutical companies and research facilities.
eCOAs is envisaged to hold ~45% of the overall spending share by the end of 2029, in line with the growing use of smartphones and tablets to seamlessly collect clinical data for analysis. Additionally, reduced cost and high scalability of eCOA are the primary determinants that continue to push its adoption during clinical trials, which is envisaged to contribute significantly to the growth of the eCOA market
Stakeholders Eyeing Opportunities through Collaborations
As the healthcare sector delves deeper into advanced technology, a large number of companies have formulated unique strategies to remain unaffected by the curve of change. Several pharmaceutical companies, research organizations and CROs have placed their focus on collaborations, while they embrace digitization to create patient-centric experiences. For instance, Signant Health has launched a partner program to collaborate with Clinical Research Organizations (CROs) that are dedicated towards improving clinical trials using patient-centric technology.
Additionally, Iqvia has recently launched a novel eCOA cloud based technology platform to quantify the patient experience, while increasing efficiency and reducing timelines at the same time.
Full Access of this Exclusive Report is Available at- https://www.factmr.com/checkout/4042
Growing Demand for Clinical Trials to Underpin Spending of Emerging Regions
As per the Fact.MR study, the increasing prevalence of various chronic infectious diseases has led to a dire unmet need for efficient clinical trials in developing countries. Moreover the rising medical expense in these regions, which can be attributed to the fact that private hospitals are the key healthcare providers herein, continue to fuel clinical trial participation in developing countries. This, coupled with the growing awareness about the cost and efficiency benefits of using eCOA, eSource & clinical trial solutions for conducting clinic trials, and comparatively lenient regulatory framework are likely to add to the lucrativeness of developing countries, such as China and India for market players.
Developed Regions Spearhead in Clinical Trials Deployment Rate
The rapidly advancing healthcare infrastructure, fast internet connectivity, and stringent regulatory product approval framework continue to make North America and Europe a hotbed of opportunities for stakeholders. With an increased number of companies getting cloud based (SaaS) deployment of clinical trial solutions in developed regions, owing to smooth internet connectivity, the market continues to accelerate at a steady pace. Cloud based deployment of digitized solutions accounted for 64% of the overall spending in Europe.
As per the study North America eCOA spending has been witnessing a steady growth over the years, with the US spending expected to account for ~90% revenue share in the regional eCOA spending in 2018. In view of the manifold cost and processing benefits of digitization in clinical trials, a larger number of pharmaceutical companies, contract research organizations, and education & research institutions have shifted their focus on paperless approaches. Growing adoption of clinical trial solutions, which contributed ~40% revenue share in North America eCOA, eSource, and clinical trials market, continue to create a window of opportunities of growth for market players.
Fact.MR study provides the long-term outlook of the eCOA, eSource, & clinical trials market for 2019 to 2029. The eCOA, eSource, & clinical trials market is envisaged to register a CAGR of ~14% through 2029.
Read More Trending Reports of Fact.MR-
Key Question answered in the survey of Global eCOA market report:
- Sales and Demand of Global eCOA
- Growth of Global eCOA Market
- Market Analysis of Global eCOA
- Market Insights of Global eCOA
- Key Drivers Impacting the Global eCOA market
- Which are the Key drivers impacted by Global eCOA market
- Restraints Shaping Market Growth
- Market Survey of Global eCOA
More Valuable Insights on Global eCOA Market
Fact.MR, in its new report, offers an unbiased Market Analysis of Global eCOA, Sales and Demand of Global eCOA, analyzing forecast statistics through 2019 and beyond. The study reveals growth projections on the basis of various criteria.
Explore Fact.MR’s Comprehensive Coverage on Healthcare Domain:
Fluoro Enzymatic Assays Market – Global Industry Analysis and Opportunity Assessment 2021 – 2031
Shower Chairs Market – Global Industry Analysis and Opportunity Assessment 2021 – 2031
Infrared Thermometer Market – Global Industry Analysis and Opportunity Assessment 2021 – 2031
About Us:
Market research and consulting agency with a difference! That’s why 80% of Fortune 1,000 companies trust us for making their most critical decisions. While our experienced consultants employ the latest technologies to extract hard-to-find insights, we believe our USP is the trust clients have on our expertise. Spanning a wide range – from automotive & industry 4.0 to healthcare & retail, our coverage is expansive, but we ensure even the most niche categories are analyzed. Our sales offices in United States and Dublin, Ireland. Headquarter based in Dubai, UAE. Reach out to us with your goals, and we’ll be an able research partner.
Contact:
US Sales Office:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
Email: sales@factmr.com
Corporate Headquarter:
Unit No: AU-01-H Gold Tower (AU),
Plot No: JLT-PH1-I3A,
Jumeirah Lakes Towers,
Dubai, United Arab Emirates